ellume covid stock symbol
Emma Court Bloomberg News. The award announced Monday provides 2318 million to Ellume USA for onshore production capacity of the at-home tests for the United States.

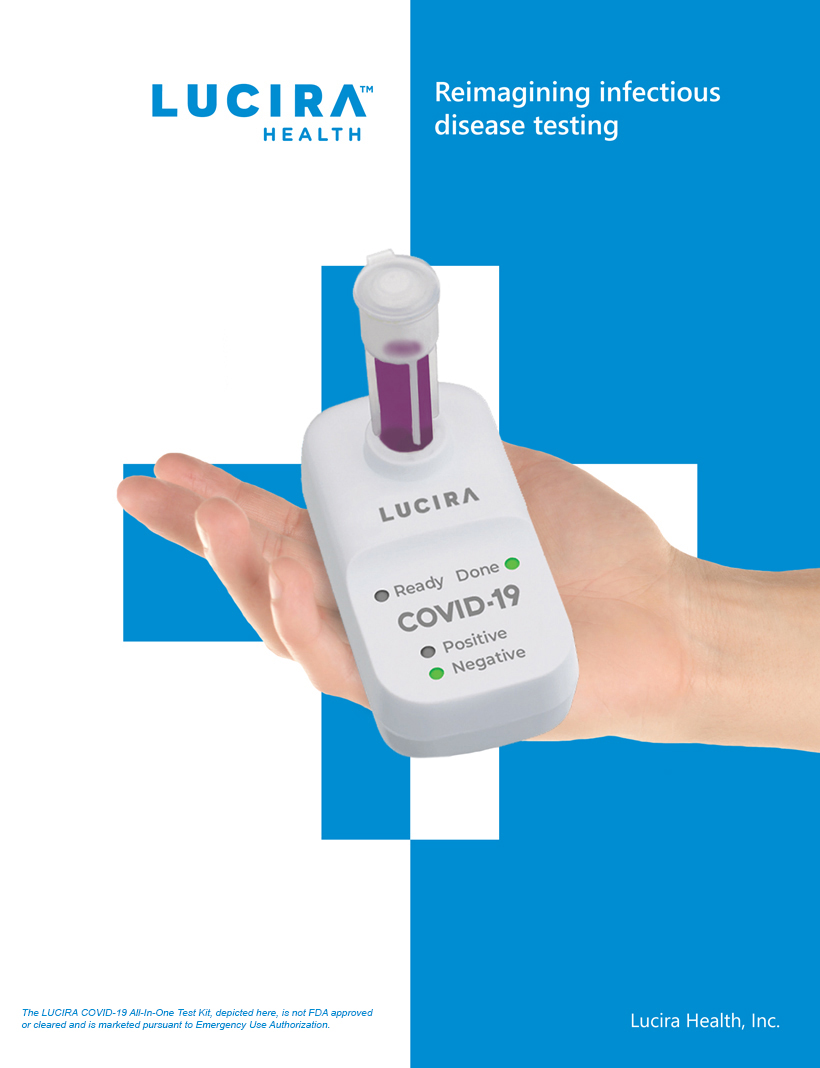
Home Ellume Covid 19 Home Test
Regulators about false positives from a startups at-home Covid-19 test the Australian company.

. Government with 85 million of its home tests. Ellumes COVID-19 Home Test the first over-the-counter OTC rapid antigen test to receive emergency use authorization from the US. The Brisbane-based Ellume has reportedly developed a highly effective COVID-19 test which has just gotten approval from the FDA.
QGEN has made a significant advancement in the field of COVID-19 testing. Food and Drug Administration. Ellumes rapid COVID-19 test was the first to receive emergency approval from the United States Food and Drug Administration FDA.
Government could boost its revenue. Government is investing 2318 million to ramp up production of at-home Covid-19 tests that dont require a prescription. Food and Drug Administration classified the recall of Ellumes over-the-counter COVID-19 home test as Class 1 the most serious type of recall after the Australian.
Bloomberg -- After complaints to US. The Ellume COVID-19 home test has demonstrated performance equivalent to or better than the rapid immunoassay tests used by doctors at the point-of-care said Dr. The Ellume COVID-19 Home Test is the only available antigen test being provided through the free distribution program that is sufficiently accessible to users who are blind or.
ABC7 New York 247 Eyewitness. Ellumes agreement with the US. The current bloodbath that stock.
White House coronavirus adviser Andy Slavitt said Monday that the government awarded a 231 million contract to scale up production of a COVID-19 home test recently. Brisbane Australia-based test-maker Ellume. As part of the contract Ellume is supplying the US.
White House COVID-19 senior adviser Andy Slavitt said the Ellume test can detect COVID-19 with 95 accuracy within roughly 15 minutes. Recently in the United States the company started commercializing a portable digital. Slavitt said this will scale up.
Stock ideas and more straight to your. White House COVID-19 senior adviser Andy Slavitt said the Ellume test can detect COVID-19 with 95 accuracy within roughly 15 minutes. One advisor reckons this biotechnology.
White House coronavirus adviser Andy Slavitt said Monday that the government awarded a 231 million contract to scale up production of a COVID-19 home test recently authorized by US. The Ellume COVID-19 Home Test is the first non-prescription over-the-counter self-test authorized by the US. Food and Drug Administration is a best-in.
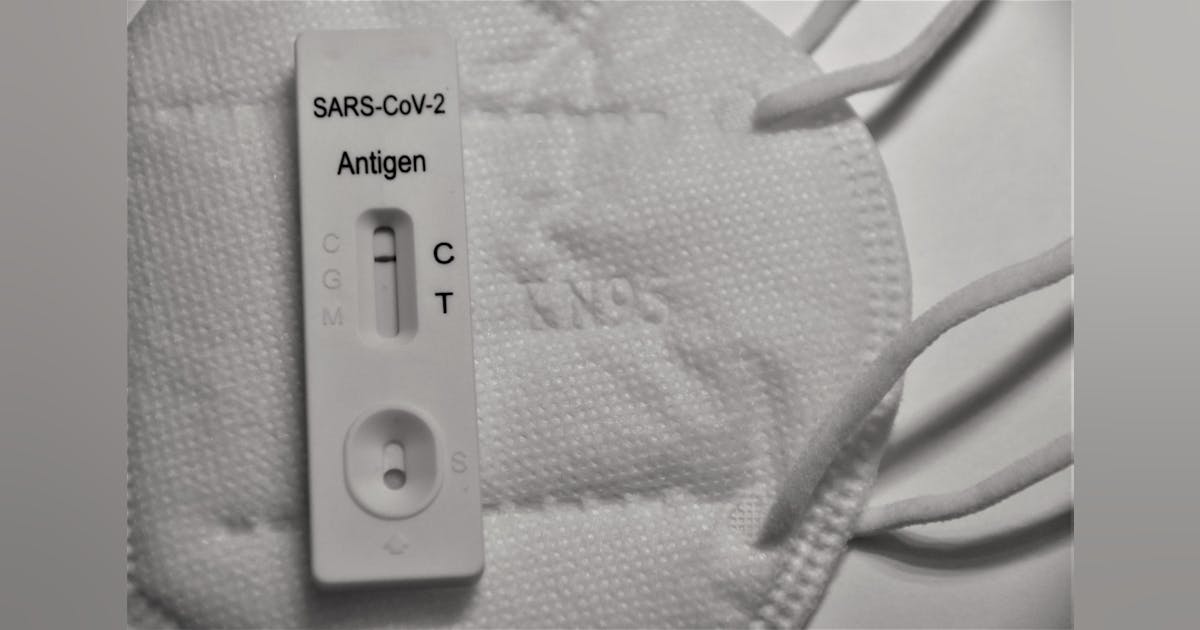
Ellume Expands Voluntary Recall Of Covid 19 At Home Test Medical Laboratory Observer
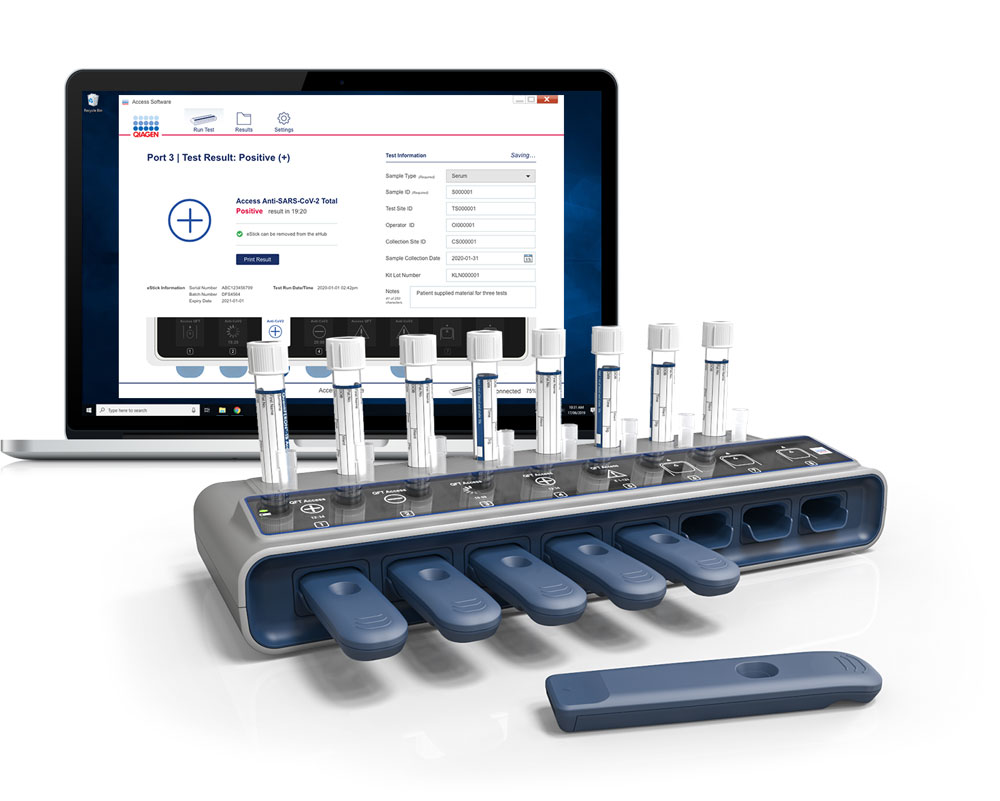
Qiagen Ellume To Build Portable Antigen Testing Hubs For Covid 19 Fierce Biotech
/cloudfront-us-east-1.images.arcpublishing.com/gray/NASVVTX7VNCUDEWHBH4NPSNEV4.jpg)
Local Shortage Of At Home Covid 19 Tests

Indiana Covid 19 Testing What To Know About At Home Covid Test Kits

Tests Vaccine Supply For Future Pandemics Requires Taxpayer Subsidies Under Biden Plan The Washington Post
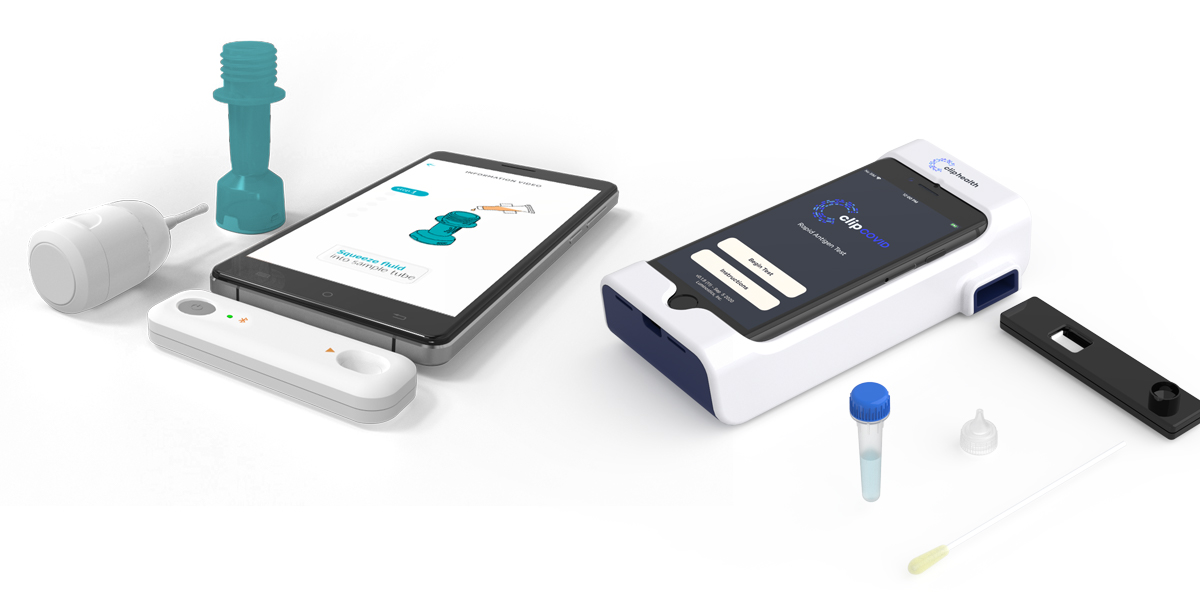
Nih Radx Initiative Advances Six New Covid 19 Testing Technologies National Institutes Of Health Nih

At Home Covid 19 Antigen Test Kits Where To Buy And What You Should Know Reviews By Wirecutter

Covid Rapid Tests To Be Sold At Cost As Joe Biden Emphasizes Testing
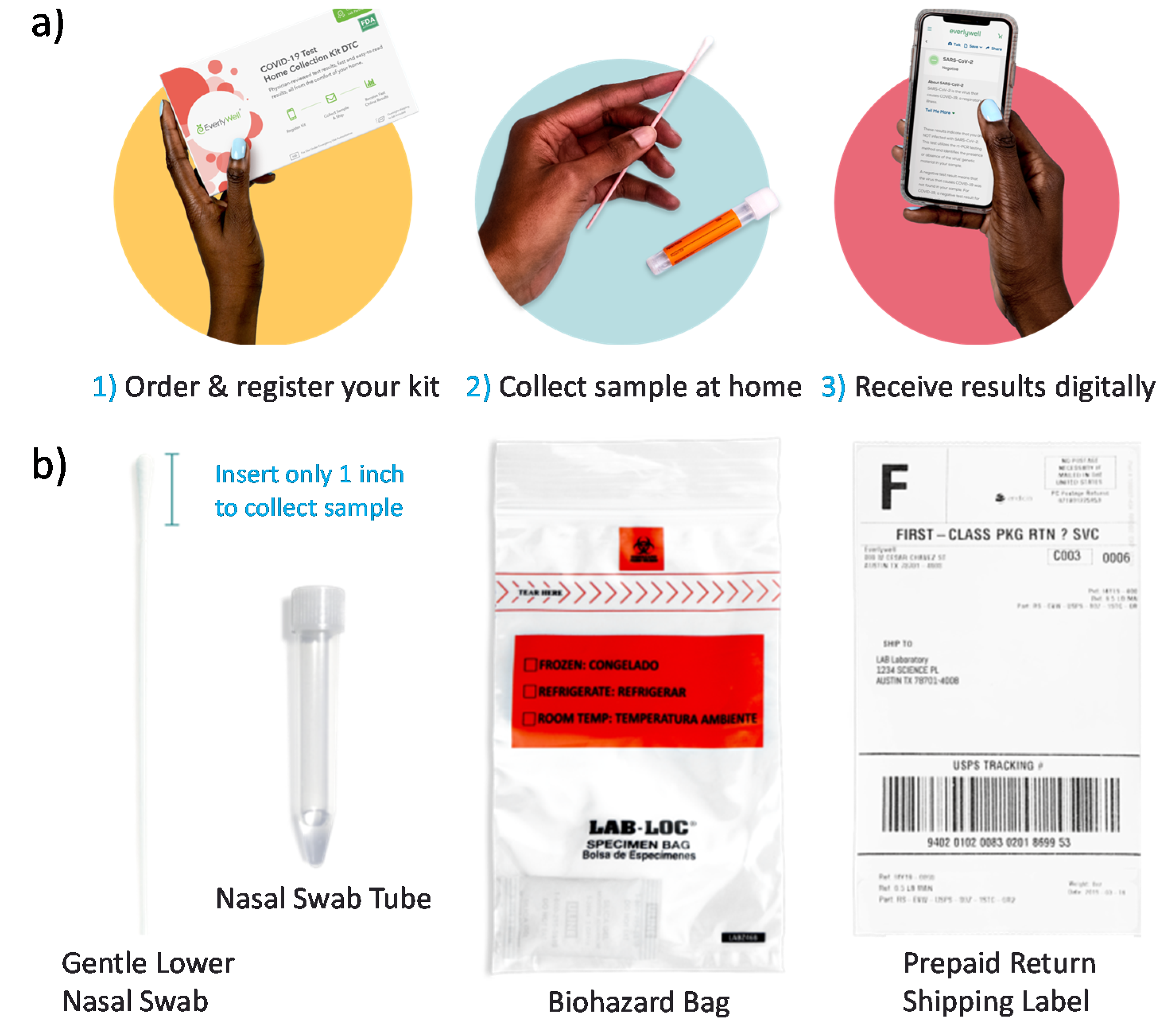
Sensors Free Full Text Covid 19 Testing And Diagnostics A Review Of Commercialized Technologies For Cost Convenience And Quality Of Tests Html

Rapid At Home Covid Tests In Short Supply Amid High Demand Los Angeles Times

Covid News Biden Commits 1 Billion To Widen Access To At Home Tests The New York Times

Amazon Com Ellume At Home Covid 19 Home Test Kit Rapid Antigen Covid Self Test Kit Results In 15 Minutes To Free Mobile App Fda Emergency Use Authorization One Pack Industrial Scientific
How To Get An At Home Rapid Covid 19 Test And How To Use Them